Epivolve Technology
We Target Human Diseases with Antibodies
Antibody therapies are an increasingly important tool for treating diseases like cancer, immune disorders, and viral infections. Therapeutic antibodies can improve human health by – among other things – directing the killing of diseased cells, delivering medicines to specific tissues, and protecting healthy cells from viruses.
To be effective as a therapy, antibodies must bind to a specific target protein. Across the exterior surface of every target protein are many sites that can be physically recognized and bound by antibodies, known as epitopes. Each epitope on a given protein can be recognized by different antibodies, but some epitopes are more immunogenic and therefore naturally attract more antibodies than others.
Until now, scientists have had to contend with the body’s natural bias towards more immunogenic epitopes. As a result, antibodies against sites on target proteins that are not inherently immunogenic have so far been difficult to find among the many other antibodies that are naturally produced by the body against more immunogenic epitopes.
Abbratech has invented a technology platform that enables the discovery of site-specific antibodies that have so far eluded discovery efforts. Now, therapies are no longer constrained by the natural bias in the body’s antibody response for certain epitopes over others, paving the way for more effective and targeted treatments.
Epivolve Enables Site-Specific Antibody Discovery
Human diseases like cancer often involve proteins that are mistakenly expressed with a single amino acid incorrectly incorporated into the full-length protein. These mutations in otherwise normal proteins can themselves be targeted by antibodies. However, conventional methods have proven limited in their ability to identify antibodies that can specifically distinguish between a mutated and healthy protein when the difference is as subtle as a single amino acid substitution.
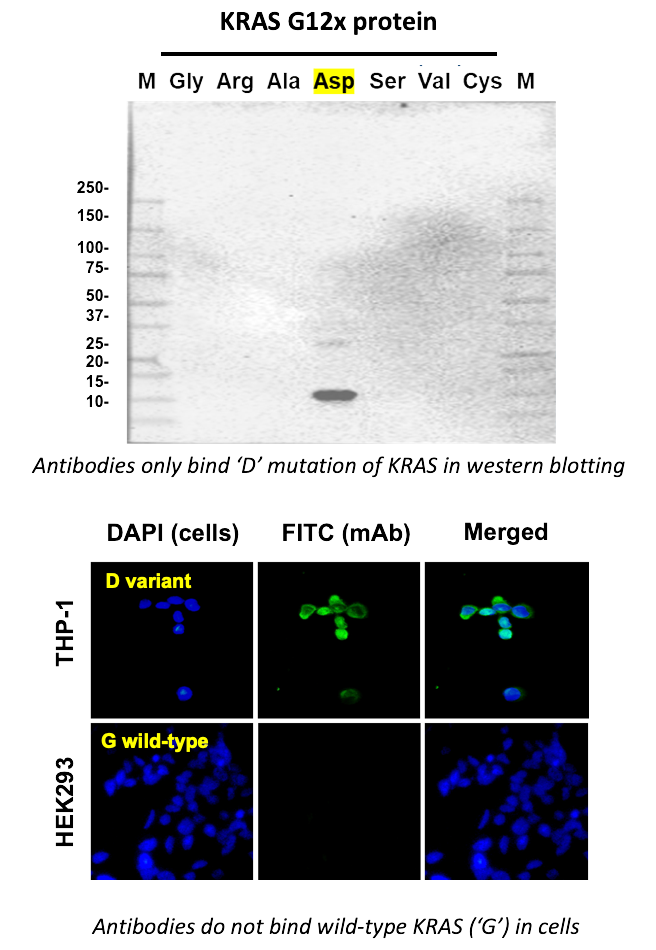
One example of a protein that – when mutated – becomes a driver of certain cancers is the signaling protein KRAS. In the case of KRAS, the 12th amino acid in the protein sequence (normally a glycine) can occasionally be mutated to an aspartate. This so-called KRAS-G12D mutation is associated with 40% of pancreatic and 27% of colorectal cancers.
To demonstrate the power of Epivolve, we generated antibodies with the ability to specifically bind only to KRAS-G12D, but not the wild-type (G12G) or any other known mutations at this position. These results demonstrate that Epivolve is capable of generating antibodies with the ability to discriminate proteins differing by as little as a single amino acid.
Epivolve Overcomes Low Immunogenicity
Some epitopes are more likely to be targeted by antibodies because they are more hydrophobic or electrically charged. These are called “dominant epitopes.” Finding antibodies for less common epitopes is hard because there are many more antibodies that target the dominant ones, making the rarer ones difficult to discover.
AbbraTech’s Epivolve technology is uniquely able to increase the frequency of finding antibodies against even low immunogenicity epitopes. As a result, therapeutic antibodies against these targets are no longer as rare as they would otherwise be.

Epivolve Overcomes Immune Tolerance
To prevent the immune system from accidentally targeting the host (as with autoimmunity), it is designed to avoid raising antibodies against “self” proteins (those naturally occurring within the body). Unfortunately, the proteins of animals used to generate therapeutic antibodies can often be identical in sequence to human proteins. In these cases, animals will not naturally raise an antibody response.
AbbraTech’s Epivolve technology is uniquely able to break immune tolerance, raising antibody responses even against targets that are identical in sequence to naturally occurring self proteins. Consequently, more human proteins are now tractable targets for antibody therapies.

Epivolve Enables Structure-Driven Exploration
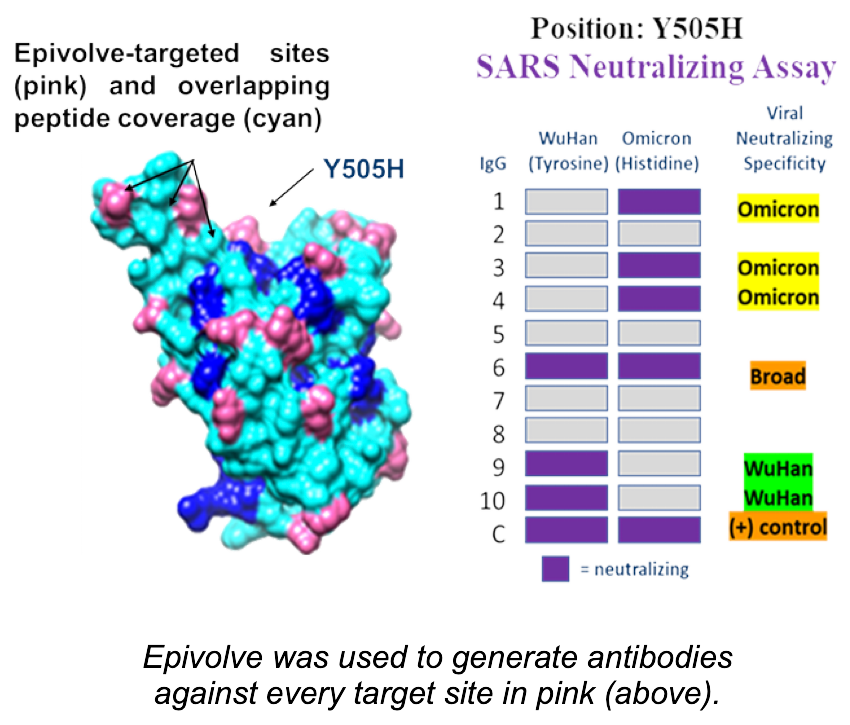
With AbbraTech’s Epivolve technology, nearly any epitope on a protein’s surface can be easily targeted by antibodies. As a result of the reduced screening effort required to find these antibodies, it is now tractable to explore epitopes across the surface of your target protein, rationally and systematically testing structure-guided hypotheses.
In the case of the spike protein for SARS-CoV-2, the virus that causes COVID-19, Epivolve was used to generate antibodies against a series of exposed epitopes in order to empirically determine which antibodies were capable of neutralizing various mutations of the virus, including the Omicron variant that evaded most approved neutralizing antibody therapies. As a result of this novel approach, the 505 position of the spike protein was shown to be capable of broadly neutralizing the original and mutated forms of the virus that causes COVID-19.